Abstract
The generation of hematopoietic stem and progenitor cells (HSPCs) from induced pluripotent stem cells (iPSCs) provides an extraordinary tool for hematological disease modeling of rare disorders such as Down syndrome (DS) associated transient myeloproliferative disorder (TMD). TMD is a preleukemic condition observed in 10-20% of children with trisomy 21 possessing the pathognomonic mutation in the transcription factor GATA1. Hematopoiesis in the bone marrow (BM) is affected by cell-cell and cell-matrix interactions. The current methods for iPSC differentiation into HSPCs utilize either 2-dimensional (2D) monolayer of mouse stromal cells or animal tissue derived extracellular matrices. Generation of a 3-dimensional (3D) culture environment attempts to facilitate both cell-cell and cell-matrix interactions during iPSC differentiation. This study reports the development of a 3D culture system for hematopoietic differentiation of iPSCs to model TMD.
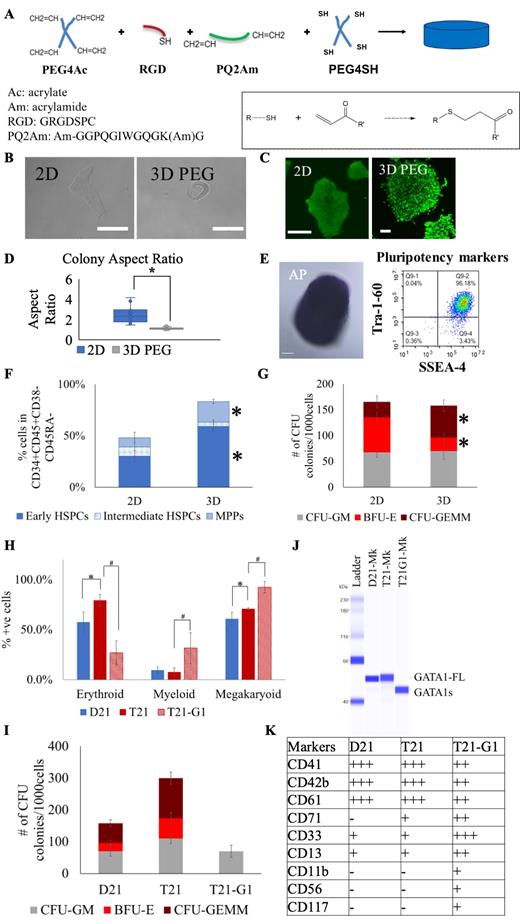
iPSC colonies were encapsulated in 3D polyethylene glycol (PEG) based hydrogels containing synthetic integrin binding peptide (GRGDSPC) and enzymatically degradable peptide (GGPQGIWGQGKG) (Fig. 1A) and cultured in maintenance medium (mTeSR1™, Stem Cell Technology) without feeder cells. There were notable morphological differences between the 3D encapsulated and 2D cultured iPSC colonies (Fig. 1B). The 3D encapsulation did not have an adverse effect on the viability of the iPSC colonies evaluated by in situ staining with viability dye (Fig. 1C). The 3D encapsulated colonies were more compact with a spheroid morphology in PEG whereas colonies in 2D were more flattened (Fig. 1D). The pluripotency of the 3D encapsulated iPSCs was confirmed alkaline phosphatase staining (purple colonies) and by the presence of >96% population expressing pluripotency markers, Tra-1-60 and SSEA-4 (Fig. 1E).
To test the efficiency of the 3D model system to generate HSPCs, the encapsulated iPSCs were subjected to hematopoietic differentiation using STEMdiff Hematopoietic Kit. Following differentiation, immunophenotype analysis of single cells by flow cytometry revealed a 1.7-fold higher CD34+CD45+CD38-CD45RA- cell percentage in 3D hydrogels compared to 2D. Further delineation of sub-populations in HSPC compartment from 2D and 3D hydrogel revealed a 1.9-fold and 2.1-fold higher population of early HSPCs and multipotent progenitors (MPPs) in 3D compared to 2D respectively (Fig 1F, *P<0.05). In colony forming unit (CFU) assay, the 3D generated HSPCs gave rise to a 2.0-fold higher number of CFU-GEMM (granulocyte, erythrocyte, monocyte, megakaryocyte) colonies compared to 2D, with 2.0-fold decreased number of BFU-E (erythroid) colonies and a similar number of CFU-GM (granulocyte, macrophage) colonies (Fig. 1G). Thus, the low modulus synthetic matrix promoted hematopoietic differentiation producing higher percentage of early HSPCs as compared to the 2D culture system.
We used this 3D system to model TMD by utilizing isogenic iPSCs with disomy 21 (D21), trisomy 21 (T21), and trisomy 21 bearing pathologic mutation in GATA1 (T21-G1). The megakaryoid population in the HSPCs generated by hematopoietic differentiation of 3D encapsulated iPSCs was characterized by the percentage of CD34+CD41+ population within the total CD41+ population, myeloid population as CD18+CD45+ and erythroid population as CD71+CD235+. T21 HSPCs showed increased erythroid and megakaryoid populations as compared to isogenic D21, consistent with the role of trisomy 21 in perturbing hematopoiesis. T21-G1 had elevated megakaryoid (93±6% vs 71±1%,) and myeloid (32±16% vs 8±4%) populations with reduced erythroid (27±12% vs 79±6%) population as compared to T21 HSPCs implicating GATA1s in altered hematopoiesis (Fig. 1H). T21-G1 HSPCs only produced CFU-GM colonies as compared to a high number of CFU-GEMM and BFU-E in T21 and D21 HSPCs (Fig. 1I). The expression of GATA1s in T21-G1 megakaryoid population was confirmed (Fig. 1J). The immunophenotype marker analysis of T21-G1 megakaryoid blasts showed expression of megakaryoid/erythroid antigens (CD41, CD61, CD42b, CD71) along with myeloid markers (CD11b, CD33, CD13) and increased expression of CD56 and CD117 consistent with TMD patients (Fig. 1K).
In conclusion, our cost-effective tunable 3D hydrogel system promoted hematopoietic differentiation of iPSCs and generated TMD model mimicking the salient features of the disease.
Barwe: Prelude Therapeutics: Research Funding. Gopalakrishnapillai: Geron: Research Funding.